Abstract
Background
Anemia in chronic kidney disease (CKD) patients develop primarily due to iron and erythropoietin deficiency, factors contributing to this include uremia induced inhibition of erythropoiesis, shortened red cell survival and nutritional deficiency. Erythropoietin stimulating agents (ESA) used in CKD increases iron demand for the production of new red blood cells (RBCs). Oral iron does not provide a reliable route to meet the demand of the iron required and poses the risk of side effects. The intravenous formulation provides the fastest route, although multiple intravenous formulations are available they require multiple dosing and still carry a risk of an adverse event. Ferric derisomaltose/iron isomaltoside (IIM) is an intravenous formulation of iron recently approved by the Food and drug administration (FDA) for patients intolerant to oral iron and non-dialysis dependent CKD patients. FDI is administered in high dose usually in a single administration. In this systematic review, we evaluated the published literature for the efficacy and safety of ferric derisomaltose in both dialysis-dependent and dialysis independent patients.
Material/Methods
A literature search was done using the following databases: PubMed, Cochrane, Embase, Clinical trials.gov, and Web of Science. The search was completed without using any filter and we used the Mesh Terms for "anemia", "iron deficiency anemia" "chronic kidney disease" and "ferric compounds". Total of 316 which were further screened and we included 2 trials, and 2 prospective studies. Case reports, preclinical trials, meta-analyses, and review articles were excluded. We followed the PRISMA guidelines for literature search and selection of studies. We only included the trial studies that were completed. We excluded the studies that did not report efficacy and safety.
Results
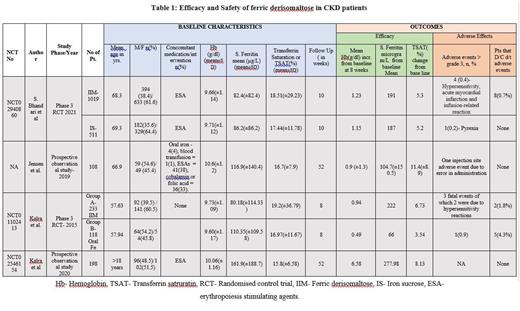
In total, amongst the four studies, 1558 CKD patients received ferric derisomaltose in a single dose. Patients in all stages of CKD were studied but most patients were between stages I to stage III CKD, stage IV and V(dialysis-dependent) were studied by Kalra et al (2020). A single dose of 1000 mg/dl of IIM, was administered intravenously. The mean pre-treatment haemoglobin level varied from 9.6g/dl to 10.6 g/dl. The mean serum ferritin ranged from 82.4 µg/L to 161.9 µg/L and the mean transferrin saturation ranged in patients from 15.8% to 18.51%. IIM receiving patients were compared with iron sucrose receiving patients by Bhandari et al, and oral iron by Kalra et al (2015). The remaining two studies compared single IIM with multiple IIM dosages. The results are summarised in the table below.
Efficacy: The increase in haemoglobin ranged between 0.9 g/dL to 6.58 g/dL from baseline measured at 8 weeks. At the same time, serum ferritin showed an increase in baseline serum ferritin from 104 µg/L to 277 µg/L and transferrin saturation (TSAT) increased in the range of 5.3%-11.4 %. All markers of iron deficiency showed significant improvement. It achieved a greater Hb response irrespective of ESA treatment in both haemodialysis dependent and non-dependent patients. In the comparison group by Bhandari et al, iron sucrose receiving patients also had similar efficacy as IIM. On the other hand, those receiving oral iron in the study by Kalra et al (2015) had much poorer efficacy as compared to IIM
Safety: Severe adverse effect was seen in a total of 10 (0.6%) patients who developed hypersensitivity reaction, acute myocardial infarction and injection site-related adverse effects with hypersensitivity being the most common. 15 (0.9%) patients discontinued the treatment. 21 (1.3%) died, and the death was attributed to end-stage renal disease.
Conclusion
Ferric derisomaltose is an effective parenteral iron therapy to treat iron deficiency when compared to oral iron therapy, however, its efficacy is very similar to other parenteral iron formulations. In terms of safety, being used as a single dose it relatively has lesser adverse events but can seldom lead to discontinuation of the drug. This drug can be recommended for iron therapy especially considering single-dose administration but more studies need to be carried out in comparison to other parenteral formulations to further clarify its efficacy in dialysis-dependent CKD patients.
Anwer: BMS / Celgene: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Janssen pharmaceutical: Honoraria, Research Funding; Allogene Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal